Abstract
Introduction While several novel therapies have been approved for the management of acute myeloid leukemia (AML), the most effective approach for the treatment of TP53-mutated (m) AML remains unclear. We previously reported on the outcome from a large cohort of patients (pts) with TP53m AML with evolving frontline therapies (Badar et al. Am J Hematol. 2022) and observed that the only intervention associated with improved survival was allogeneic stem cell transplantation (allo-HSCT). In this report, we specifically analyze the characteristics and outcome of pts with TP53m AML following allo-HSCT.
Method The study was conducted through the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND), a collaboration involving 10 US academic institutions. Among 368 pts with TP53m AML diagnosed between 2012-2021, a total of 49 pts received allo-HSCT after first line therapy [1L] and 20 after second line therapy [2L]). We analyzed factors predicting survival among these pts. Event-free survival (EFS) was estimated from time of diagnosis/progression after first line therapy to progression or death after allo-HSCT in 1L and 2L group, respectively. Overall survival (OS) was calculated from the date of therapy (1L or 2L) until last follow up or death.
Result Baseline characteristics
The median age of pts who received allo-HSCT after 1L was 63 years (yrs) (range [R] 33-75) and after 2L was 61 yrs (R, 18-68) (Table 1). Similar proportion of pts had secondary AML (19% and 25%) and had complex cytogenetic (84% and 75%) in the 1L and 2L groups, respectively. A higher proportion of pts (37% and 45%) received intensive chemotherapy (3+7 or high dose cytarabine) followed by venetoclax based regimen (24.5% and 30%) in the 1L and 2L groups, respectively. Remaining patient in both groups received hypomethylating agent-based therapy or other low intensive therapy prior to allo-HSCT (Table 1).
Responses prior to allo-HSCT
Seventy five percent of patients (n= 37) were in complete remission with or without count recovery (CR/CRi) among them 70% (n=26) were minimal residual disease (MRD) negative in 1L group, whereas 80% (n= 16) were in CR/CRi (MRD -ve 7 [43%]) in 2L group receiving allo-HSCT.
Allo-HSCT characteristics and day 100 response
Forty percent received myeloablative conditioning (MAC) and 57% received reduced intensity conditioning in 1L group, similarly 40% and 60% received MAC and RIC in 2L group, respectively. Fourteen percent received related donor (MRD), 60% received unrelated donor (MUD), 20% received haploidentical (haplo) and 3% received cord blood in 1L group. Similarly, 40%, 44% and 22% received MRD, MUD and haplo in 2L group, respectively. Thirty one percent and 50% had acute graft versus host disease (GVHD) in 1L and 2L group, respectively. Forty and 44% had cGVHD in 1L and 2L group, respectively. 88% and 50% pts were in CR/CRi at day 100 assessment in 1L and 2L group, respectively.
Survival
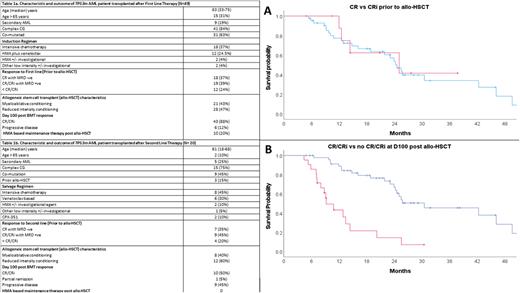
The median EFS in the 1L group was 23.5 (95% confidence interval [CI]: 10.75-36.24) months in the 2L group was 8.93 (95% CI: 2.70-15.16) months. The median OS in the 1L group was 46.1 months (95% CI: 17.4-74.7) and in the 2L group was 18.2 months (95% CI: 7.05-29.4). In sub-group analysis median OS in pts who were in CR vs CRi prior to allo-HSCT (24.5 vs 25.0 months [mo], p=0.84 [Figure 1A]), received MAC vs RIC conditioning (24.2 vs 25.0 mo, p= 0.61), had aGVHD (25.0 vs 21.8 mo, p= 0.71) or received HMA based maintenance therapy post allo-HSCT (24.2 vs 21.8 mo, p=0.59) was not significantly different. Conversely, median OS was significantly better in pts who were in CR/CRi vs progressive disease at day 100 assessment post allo-HSCT (30.5 vs 9.2 mo, p= <0.001, [Figure 1B]) or had cGVHD vs no cGVHD (46.10 vs 12.87 mo, p= <0.001).
Conclusion Our report suggests that allo-HSCT is associated with improved long-term outcomes among pts with TP53m AML. Outcome was significantly better among patients who were in complete remission at day 100 post allo-HSCT or had cGVHD. Although prospective studies are needed to be definitive, our analyses demonstrate that alloHCT should be offered to eligible patients with TP53m AML.
Disclosures
Atallah:Novartis: Consultancy, Research Funding; Takeda: Research Funding; BMS: Consultancy, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Blueprint: Speakers Bureau. Shallis:Bristol Myers Squibb and Gilead Sciences, Inc: Honoraria; Gilead Sciences, Inc.: Honoraria. Stahl:Novartis: Other: Advisory Board; Boston Consulting: Consultancy; Curis Oncology: Consultancy. Patel:Kronos Bio: Research Funding; Servier/Agios: Research Funding; Pfizer: Research Funding; Celgene/BMS: Research Funding; AbbVie: Consultancy. Abaza:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; ALX Oncology: Research Funding. Duvall:Jazz Pharmaceuticals: Consultancy. Palmisiano:Genentech: Research Funding; AbbVie: Research Funding. Kota:Incyte: Honoraria; Ariad: Honoraria; Xcenda: Honoraria; Novartis: Honoraria; Pfizer Inc: Honoraria, Research Funding. Goldberg:Aprea, Aptose, AROG, Celularity, Pfizer, Prelude Therapeutics: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; DAVA Oncology: Honoraria; Genentech: Consultancy; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Moderna, Novavax: Current equity holder in publicly-traded company. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal